Knee Alignment Device
Knee Alignment Device
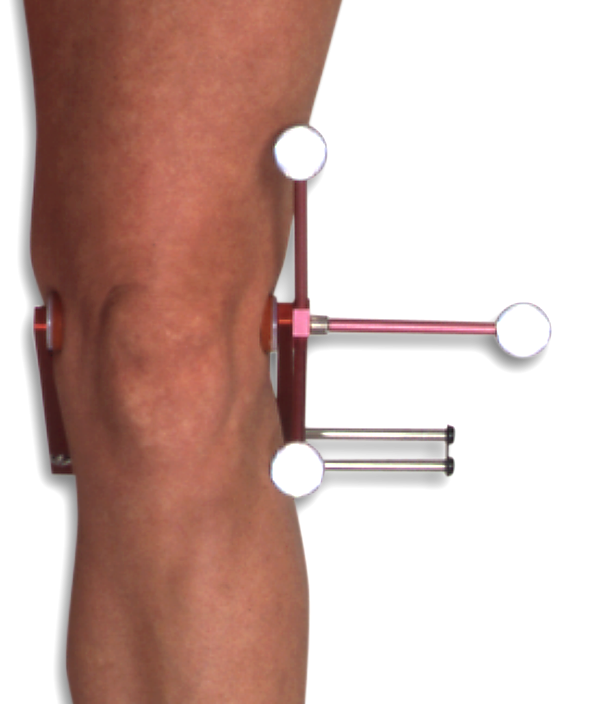
Sold as a pair of devices, the Knee Alignment Device (KAD) is used during static motion capture trials to define the the knee flexion/extension axis of the subjects knee prior to a series of walking trials. This eliminates a varus/valgus error that may result from minor marker placement errors of the thigh and shank markers during gait testing. In addition, the use of the KAD based knee model enables the subject marker set to eliminate the use of medial knee markers, which are prone to being knocked off during testing when the subject knees cross or pass closely during swing. This is primarily a problem during gait trials.
Two KAD sizes are available - the standard Adult KAD and a much smaller Kiddy-KAD designed to be a fully functional KAD with small children.
The KAD is a spring-loaded metal jig that fits gently over the subjects knee while a static calibration data collection is performed. The design of the KAD is such that the absolute distance between all three of the markers on the device is identical (5.66 inches). This enables gait analysis software that supports KAD use to establish a virtual knee marker at the central joint of the Knee Alignment Device and this, combined with the KAD wand marker, enables the knee flexion axis to be accurately measured. The KAD-01 works with knee widths from 1.5 inches up to 6 inches (4-15cm). Data collected with the Knee Alignment Devices is can be processed by the VICON Clinical Manager, Vicon Nexus Plug-in-Gait, Peak Motus, and the C-Motion Visual-3D biomechanical modeling software as well as software packages available from other 3D Motion Capture System manufacturers.
Maintenance and spare parts for the KAD are available from Motion Lab Systems.
Operation
When a software application that supports KADs finds marker labels for a Knee Alignment Device defined in the 3D data file, it will use the orientation of the three markers on the Knee Alignment Device to determine the knee flexion axis as defined by the physical therapist. After the knee flexion axis has been established, the software calculates the relative transverse alignment of this axis, to the transverse plane orientation of the thigh and shank, as calculated using the mid-thigh and mid-shank stick markers. These relative alignments are stored as Thigh and Shank Rotations and are applied to all dynamic data within the session.
Thus the correct alignment of the thigh and shank wands becomes less critical, as any minor alignment errors are measured during the static KAD trial, and can be automatically corrected during the processing of the dynamic trials. Please refer your software vendors User Guide or contact the application manufacturer for details of the kinematic calculations that utilize the KAD data.
The design of the KAD uses a 14mm retro-reflective marker - however, many 3D motion capture systems use different marker diameters and it is common for users to replace the KAD markers. This has no effect of the functioning of the KAD device.
Repair
The KAD is designed to "fail-safely" in the event of an accident - each of the three arms is attached to the KAD body by a nylon thread that will break if the KAD is dropped or the subject falls while wearing it - this protects the subject and, in general, the KAD from serious harm. Replacement nylon threads are available from Motion Lab Systems and can be easily replaced, or if you wish, you can return the damaged KAD to us for repair.
The KAD development history
Motion Lab Systems developed the first commercially available Knee Alignment Device (KAD) for use with the Vicon Clinical Manager (VCM) software from Oxford Metrics Ltd (now Vicon Motion Systems) based on the original design created at Newington Children's Hospital in Connecticut by Dennis Tyburski and Roy Davis. This prototype was constructed from various materials found around the lab (principally Delrin, glue and rubber bands) and used by the Newington Gait Analysis software to locate the knee axis.
Oxford Metrics included software support for the KAD in the Vicon Clinical Manager (VCM) software, written to reproduce the results generated by the Helen Hayes Hospital Software gait analysis environment and this created a demand for the devices which, at that point, only existed in the Newington lab. Motion Lab Systems worked with the Dennis Tyburski to create a version of the KAD that would be easy to manufacture, reliable and lightweight. These devices were then sold by Oxford Metrics with their VCM software - the MLS units are the original, red, anodized, aluminum devices that are illustrated above.
In 1994 Oxford Metrics/Vicon designed and supplied their customers with a flexible black plastic KAD of their own design but discontinued this design after a few years due to accuracy and reliability issues. Motion Lab Systems has continued to manufacture, sell, and repair, the original MLS designed KAD based on the original Newington Children's Gait Lab Hospital design.
Couldn't load pickup availability
Share